please answer in text because i dont understand handwriting
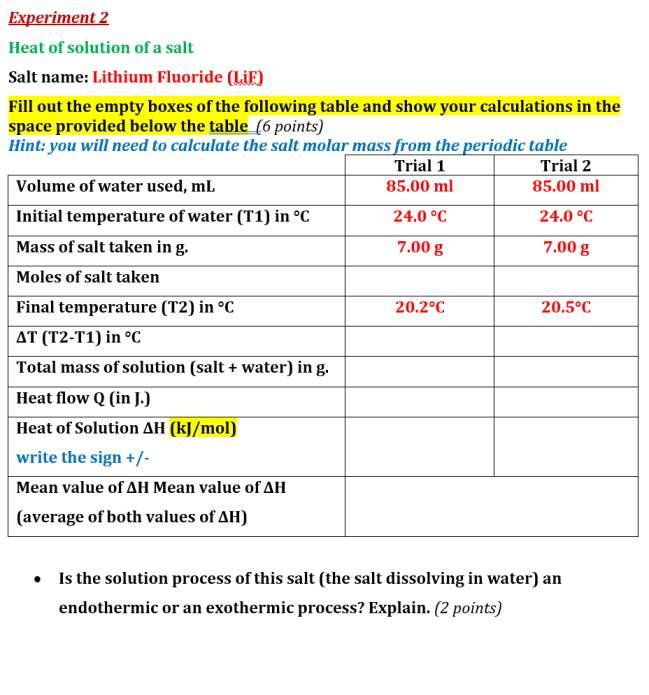
Experiment 2 Heat of solution of a salt Salt name: Lithium Fluoride (LiF) Fill out the empty boxes of the following table and show your calculations in the space provided below the table (6 points) Hint: you will need to calculate the salt molar mass from the periodic table Trial 1 Trial 2 Volume of water used, mL 85.00 ml 85.00 ml Initial temperature of water (T1) in °C 24.0 °C 24.0 °C Mass of salt taken in g. 7.00 g 7.00 g Moles of salt taken Final temperature (T2) in °C 20.2°C 20.5°C AT (T2-T1) in °C Total mass of solution (salt + water) in g. Heat flow Q in J.) Heat of Solution AH (kJ/mol) write the sign +/- Mean value of AH Mean value of AH (average of both values of AH) • Is the solution process of this salt (the salt dissolving in water) an endothermic or an exothermic process? Explain. (2 points)
没有找到相关结果