Please help me answer these, thank you

The solubility product constant(Ksp) of lead(II) iodide is 1.4 x 10 at 25°C. Calculate AG°for the dissociation of lead(II) iodide in -8 water. PbIz(s) = Pb2+ (aq) + 21+ (aq)
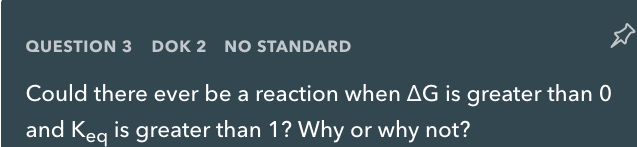
QUESTION 3 DOK 2 NO STANDARD Could there ever be a reaction when AG is greater than 0 and Keq is greater than 1? Why or why not?
没有找到相关结果