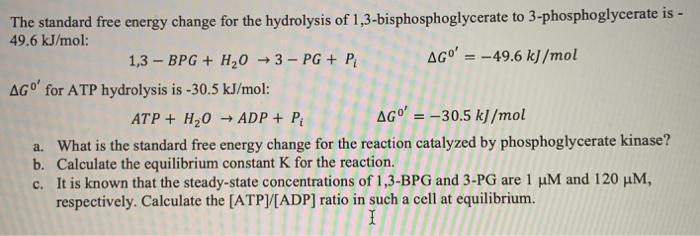
The standard free energy change for the hydrolysis of 1,3-bisphosphoglycerate to 3-phosphoglycerate is - 49.6 kJ/mol: 1,3 - BPG + H,0 →3 - PG + P AGO' = -49.6 kJ/mol AGO' for ATP hydrolysis is -30.5 kJ/mol: ATP + H2O - ADP + P AGO' = -30.5 kJ/mol a. What is the standard free energy change for the reaction catalyzed by phosphoglycerate kinase? b. Calculate the equilibrium constant K for the reaction. c. It is known that the steady-state concentrations of 1,3-BPG and 3-PG are 1 uM and 120 MM, respectively. Calculate the [ATP][ADP) ratio in such a cell at equilibrium. 1
没有找到相关结果