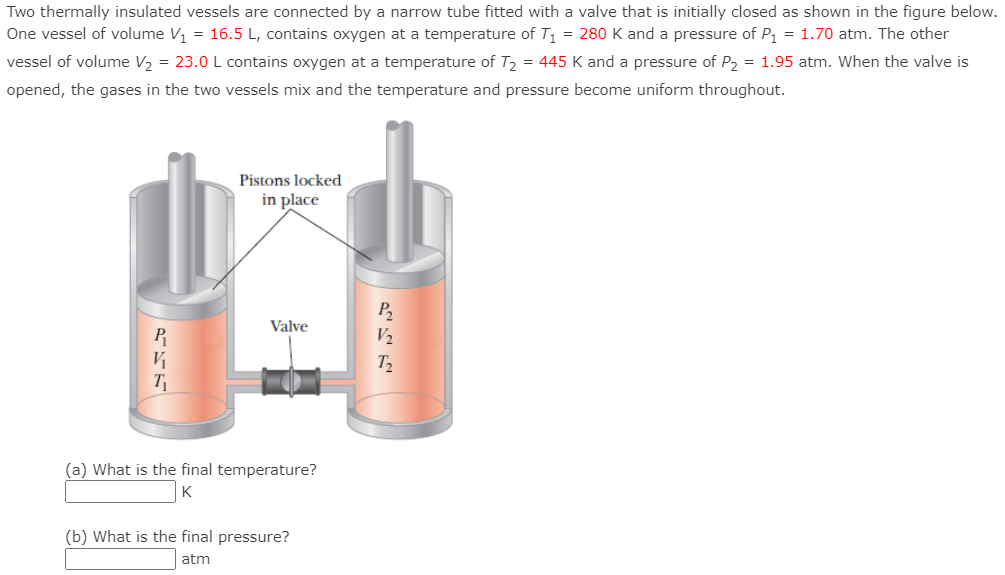
Two thermally insulated vessels are connected by a narrow tube fitted with a valve that is initially closed as shown in the figure below. One vessel of volume V1 = 16.5 L, contains oxygen at a temperature of T1 = 280 K and a pressure of P1 = 1.70 atm. The other vessel of volume V2 = 23.0 L contains oxygen at a temperature of T2 = 445 K and a pressure of P2 = 1.95 atm. When the valve is opened, the gases in the two vessels mix and the temperature and pressure become uniform throughout. Pistons locked in place Valve P2 V2 T2 V T (a) What is the final temperature? K (b) What is the final pressure? atm
没有找到相关结果