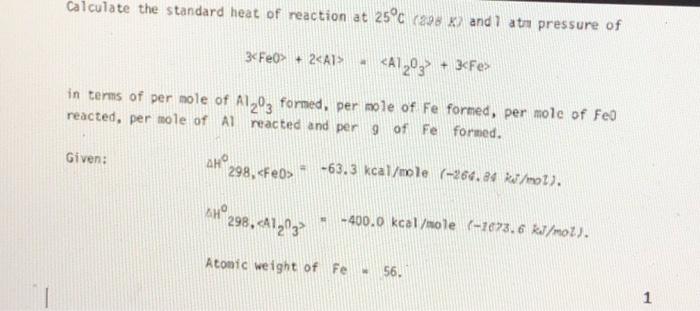Calculate the standard heat of reaction at 25°c (2 x and 1 at pressure of 3cFe0+ 2<A1 <A%203+ 3Fe> in terms of per mole of Alzºz formed, per mole of Fe formed, per mole of Feo reacted, per mole of Alreacted and per 9 of Fe formed. Giv
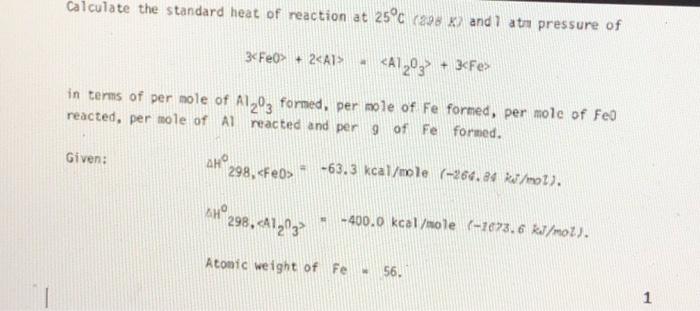
Calculate the standard heat of reaction at 25°c (2 x and 1 at pressure of 3cFe0+ 2 in terms of per mole of Alzºz formed, per mole of Fe formed, per mole of Feo reacted, per mole of Alreacted and per 9 of Fe formed. Given: AHO 298, - -63.3 kcal/mole (-264.34 kJ/mol). 44°298, A1293 - -400.0 kcal/mole (1673.6 kJ/mol). Atomic weight of Fe 56. 1