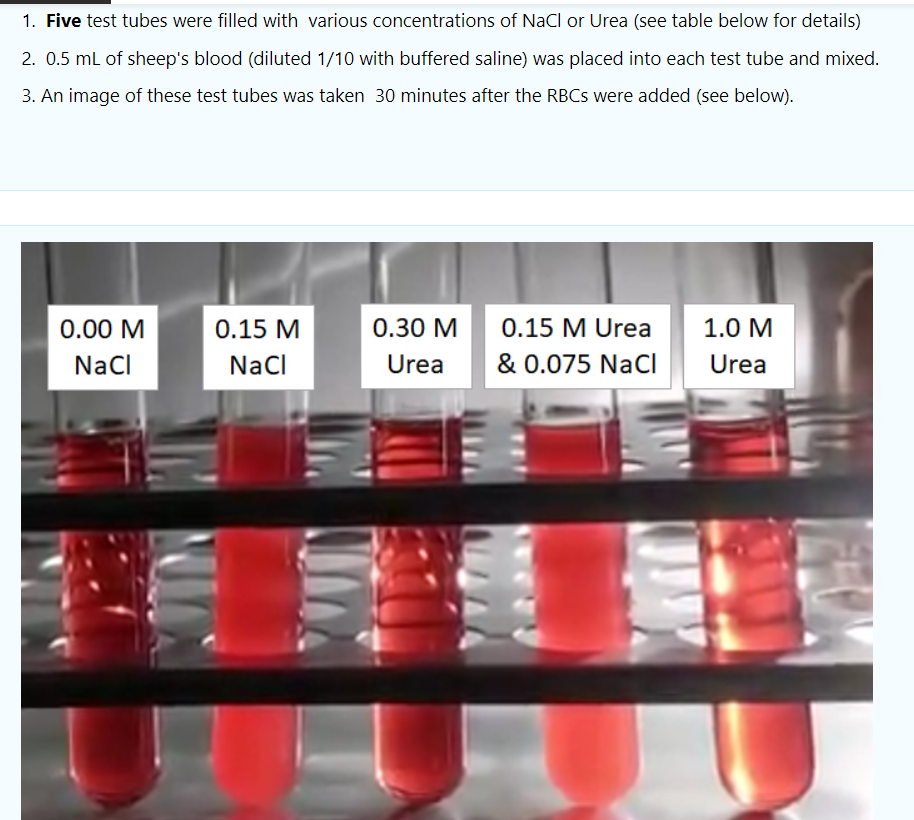
1. Five test tubes were filled with various concentrations of NaCl or Urea (see table below for details) 2. 0.5 mL of sheep's blood (diluted 1/10 with buffered saline) was placed into each test tube and mixed. 3. An image of these test tubes was taken 30 minutes after the RBCs were added (see below). 0.00 M Naci 0.15 M NaCl 0.30 M Urea 0.15 M Urea & 0.075 Naci 1.0 M Urea
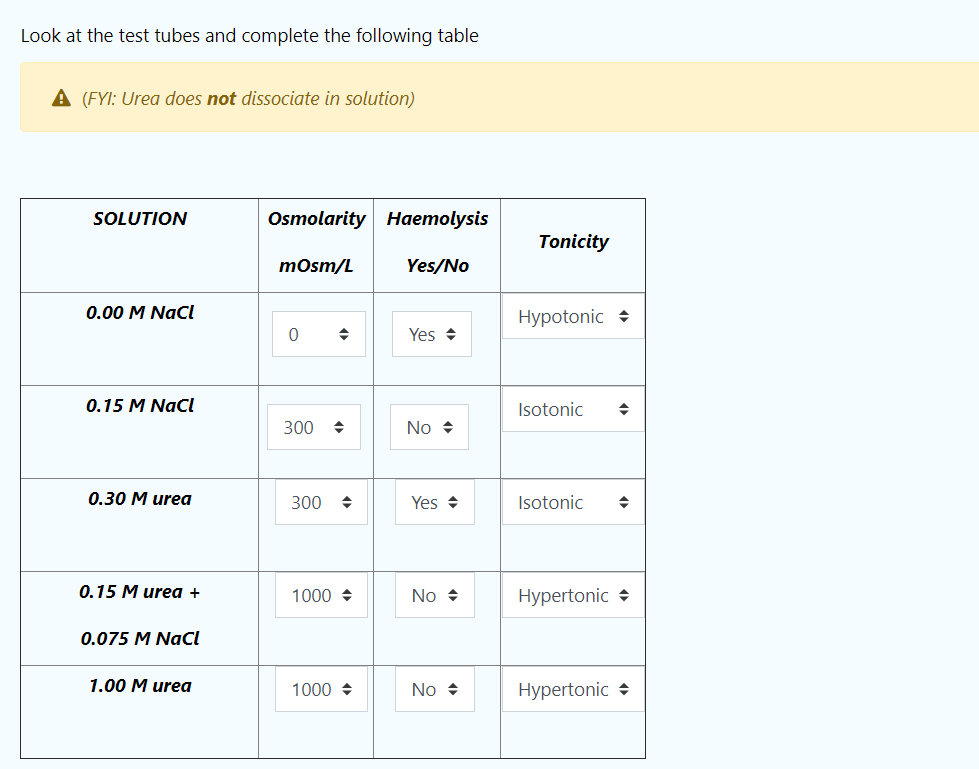
Look at the test tubes and complete the following table A (FYI: Urea does not dissociate in solution) SOLUTION Osmolarity Haemolysis Tonicity mOsm/L Yes/No 0.00 M NaCl Hypotonic 0 Yes 0.15 M NaCl Isotonic 300 No 0.30 M urea 300 Yes Isotonic 0.15 M urea + 1000 No Hypertonic 0.075 M NaCl 1.00 M urea 1000 No. Hypertonic
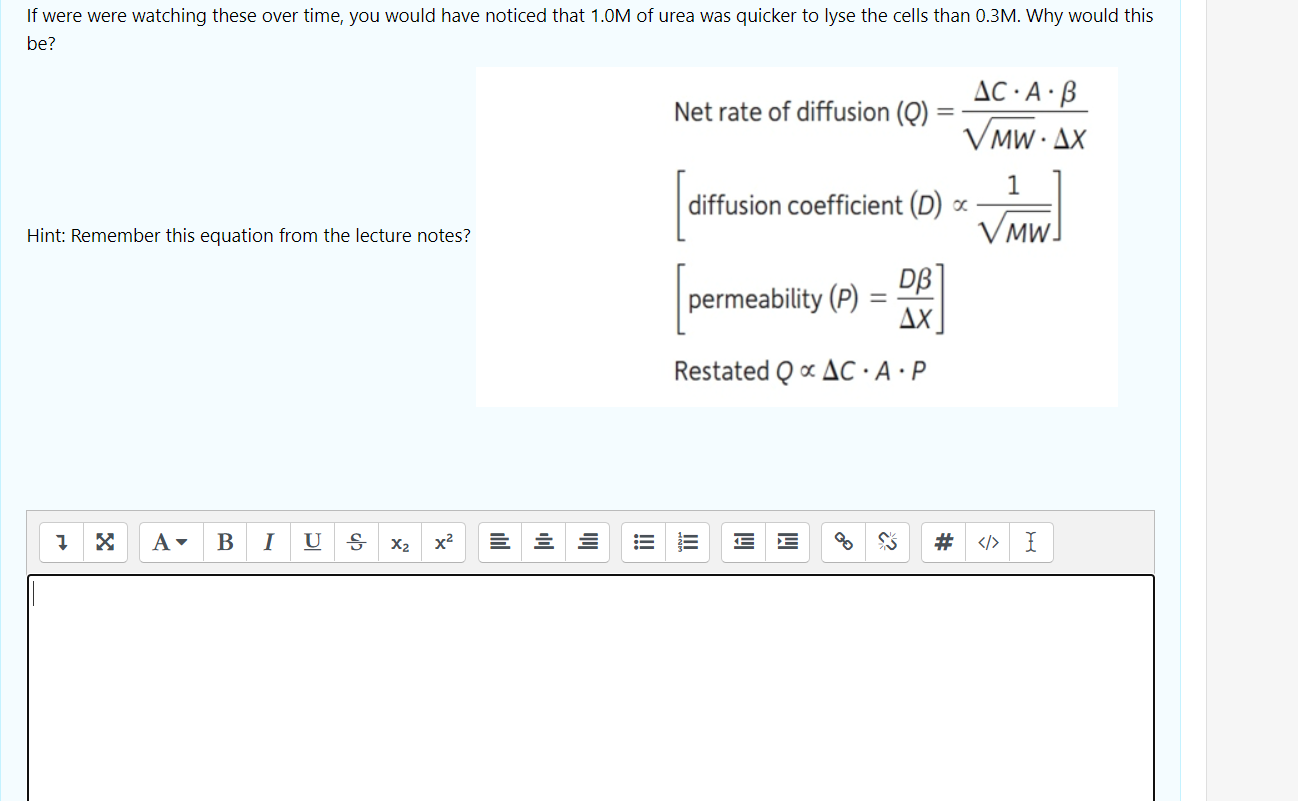
If were were watching these over time, you would have noticed that 1.0M of urea was quicker to lyse the cells than 0.3M. Why would this be? Net rate of diffusion (Q) AC:A:ß VMWAX 1 diffusion coefficient (D) : Hint: Remember this equation from the lecture notes? MW = DB permeability (P) AX Restated Q & AC · A:P 7 x A- B 1 U ab X2 x2 를 들 iii .!!! lil # I
没有找到相关结果