
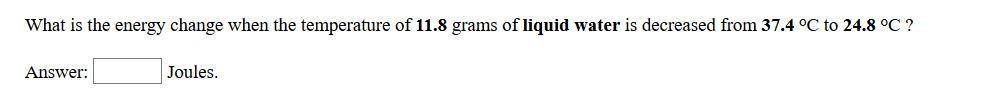
How much energy is required to raise the temperature of 10.7 grams of solid zinc from 21.9 °C to 37.1 °C ? Answer: Joules. What is the energy change when the temperature of 11.8 grams of liquid water is decreased from 37.4 °C to 24.8 °C ? Answer: Joules.
没有找到相关结果