Please Fill in the boxes when answering the question!!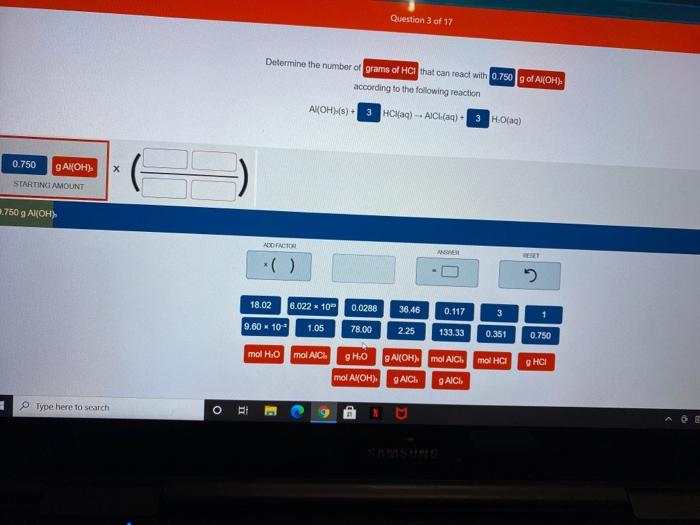
Question 3 of 17 Determine the number of grains of HCl that can react with 0.750g of Al(OH) according to the following reaction Al(OH)(s) + 3 Haq) -- AIC (aq) 3 HO(aa) 0.750 GA(OH). x STARTING AMOUNT 1.750 g AWOH) ADD FACTOR 0.0288 36.46 18.02 6.022 - 10 9.60 - 10 1.05 0.117 3 78.00 225 133.33 0.351 0.750 mol H.O mol AICI mol AICI mol HCI онСІ gHO gAl(OH) mol A OH) GAICI GAICS Type here to search
没有找到相关结果