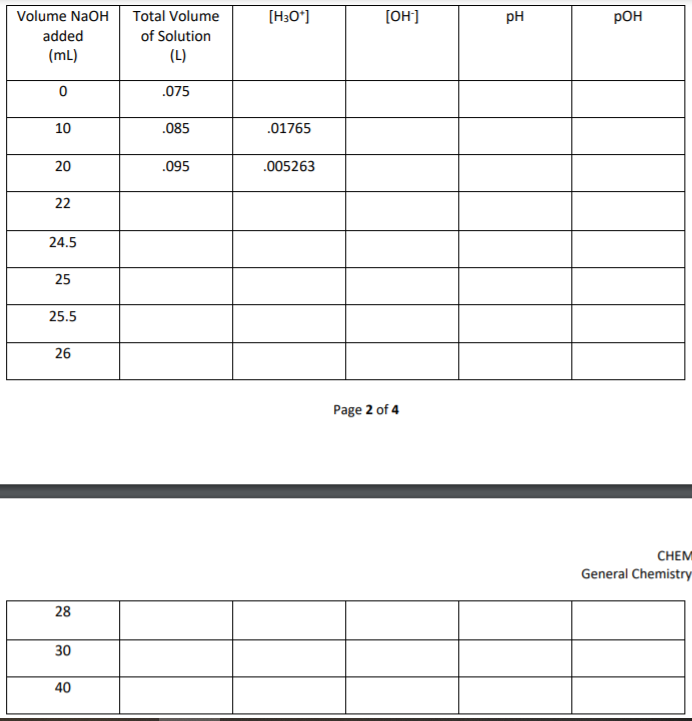
You are titrating 25 mL of 0.1 M HCL with 0.1 M NaOH. Add 50 mLof DI water to the HCL for a total volume of 75 mL of titrand.HCL(aq) + NaOH(aq) —> H2O(aq) + NaCl(aq)
Initial pH: Use the initial molarity and volumeof HCl above to calculate the new molarity of the HCl after addingDI water. Don’t forget to use liters not mL for the volume andremember the total volume is 75 mL. Alternatively, you can use M1 xV1 = M2 x V2 since adding the 50mL of water is a dilution and doesnot change the number of moles of acid. Enter this [H3O + ] in thetable under the [H3O + ] column where the volume of NaOH is zero.Calculate and enter the pH using the formula above. Now calculateand enter the pOH using the pH + pOH = 14 formula. Calculate andenter the [OH- ] using the [OH- ] = 10-pOH pH afteraddition of NaOH: After 10 mL of NaOH has been addedcalculate and enter the [H3O + ] concentration. You will do thiswith an ICE table as shown on the calculation sheet. Once you havefound this concentration calculate and enter pH, pOH, and [OH- ] asyou did in the problem above. Continue with the same calculationsthrough a volume of 24.5 mL of NaOH added. Equivalencepoint (25 mL NaOH): At equivalence, all moles of acid andbase have been neutralized. The only substances in the beaker aresalt and water. How will you calculate pH? The pH is based upon the[H3O + ] from the auto ionization of water. Set up an equilibriumexpression based on the chemical equation: CHEM 1412 GeneralChemistry II Lab Page 2 of 4 H2O(l) —> [H3O + ](aq) + [OH- ](aq) . Use Kw above to calculate the [H3O + ] and [OH- ]concentrations and then pH and pOH. After equivalence(excess NaOH): Now you will calculate the [OH- ]concentration using an ICE table as shown on the calculationsheets. Remember the moles from the first 25 mL of NaOH added andthe HCl have been neutralized. Once you have found thisconcentration calculate and enter pOH, pH, and [H3O + ] as you didin the problems above. Continue with the same calculations througha volume of 40 mL of NaOH added.
A student titrates 20 mL of a 0.5 M HCl solution with a 0.5 MNaOH. How many moles of HCl are remaining after addition of 15 mLof NaOH? What is the total volume of the solution? What is the pHof the solution? What is the pOH of the solution?
A student titrates 20 mL of a 0.5 M HCl solution with 0.5M NaOH. How many moles of NaOH are remaining after the addition of24 mL of NaOH? What is the total volume of the solution? What isthe pOH of the solution? What is the pH of the solution?
[H30*) [OH-] pH POH Volume NaOH added (mL) Total Volume of Solution (L) 0 .075 10 .085 .01765 20 .095 .005263 22 24.5 25 25.5 26 Page 2 of 4 CHEM General Chemistry 28 30 40
没有找到相关结果