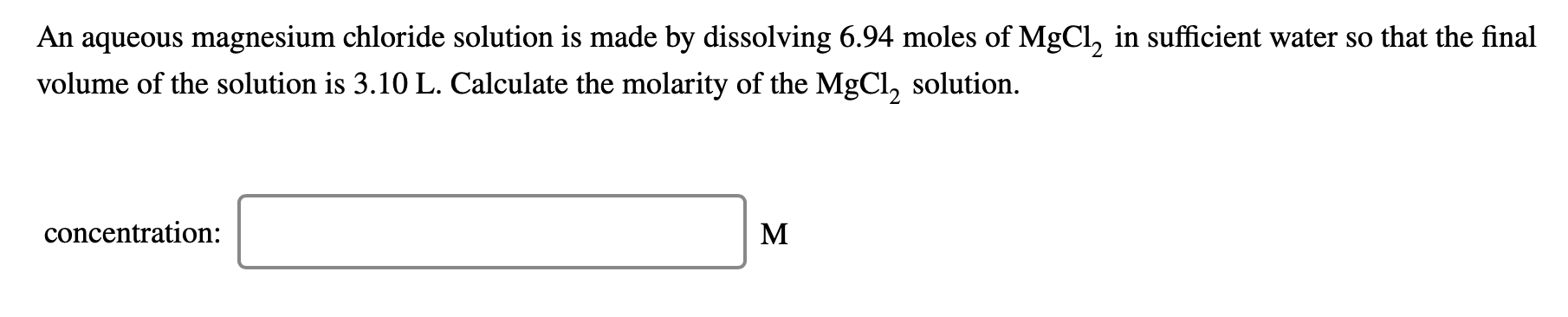
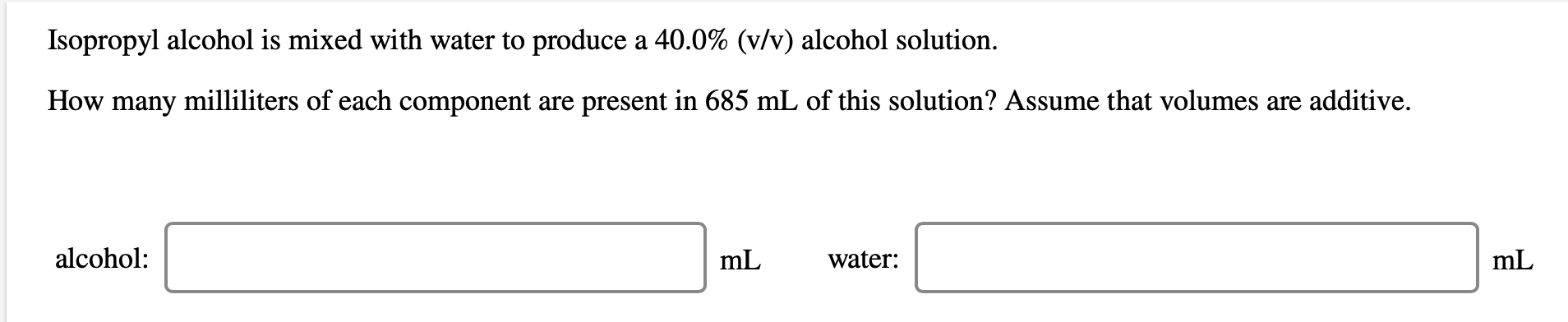
An aqueous magnesium chloride solution is made by dissolving 6.94 moles of MgCl, in sufficient water so that the final volume of the solution is 3.10 L. Calculate the molarity of the MgCl, solution. concentration: M Isopropyl alcohol is mixed with water to produce a 40.0% (v/v) alcohol solution. How many milliliters of each component are present in 685 mL of this solution? Assume that volumes are additive. alcohol: mL water: mL
没有找到相关结果