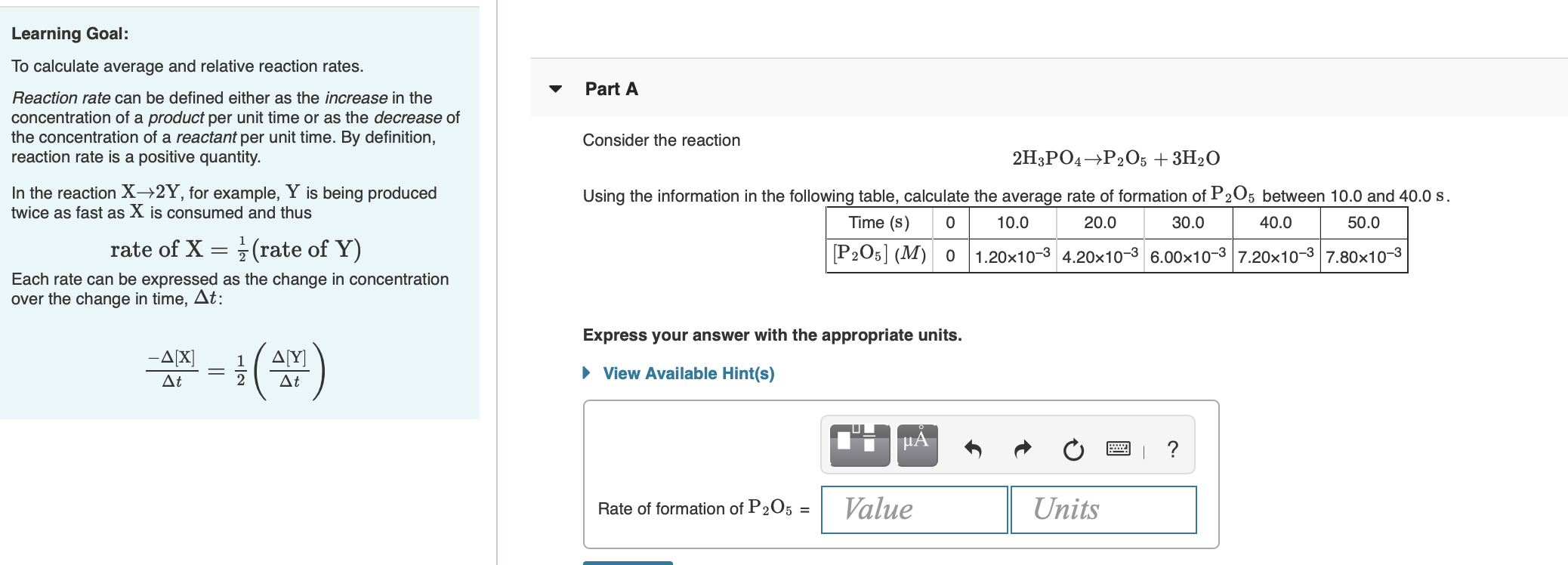
Learning Goal: Part A To calculate average and relative reaction rates. Reaction rate can be defined either as the increase in the concentration of a product per unit time or as the decrease of the concentration of a reactant per unit time. By definition, reaction rate is a positive quantity. In the reaction X2Y, for example, Y is being produced twice as fast as X is consumed and thus Consider the reaction 2H3PO4 →P205 + 3H20 Using the information in the following table, calculate the average rate of formation of P205 between 10.0 and 40.0 S. Time (s) 0 10.0 20.0 30.0 40.0 50.0 [P205] (M)0 1.20x10-3 4.20x10-3 6.00x10-3 7.20x10-3 7.80x10-3 rate of X = }(rate of Y) Each rate can be expressed as the change in concentration over the change in time, At: Express your answer with the appropriate units. -A[X] At ( Δ[Υ] At View Available Hint(s) uA ? Rate of formation of P205 = Value Units
没有找到相关结果