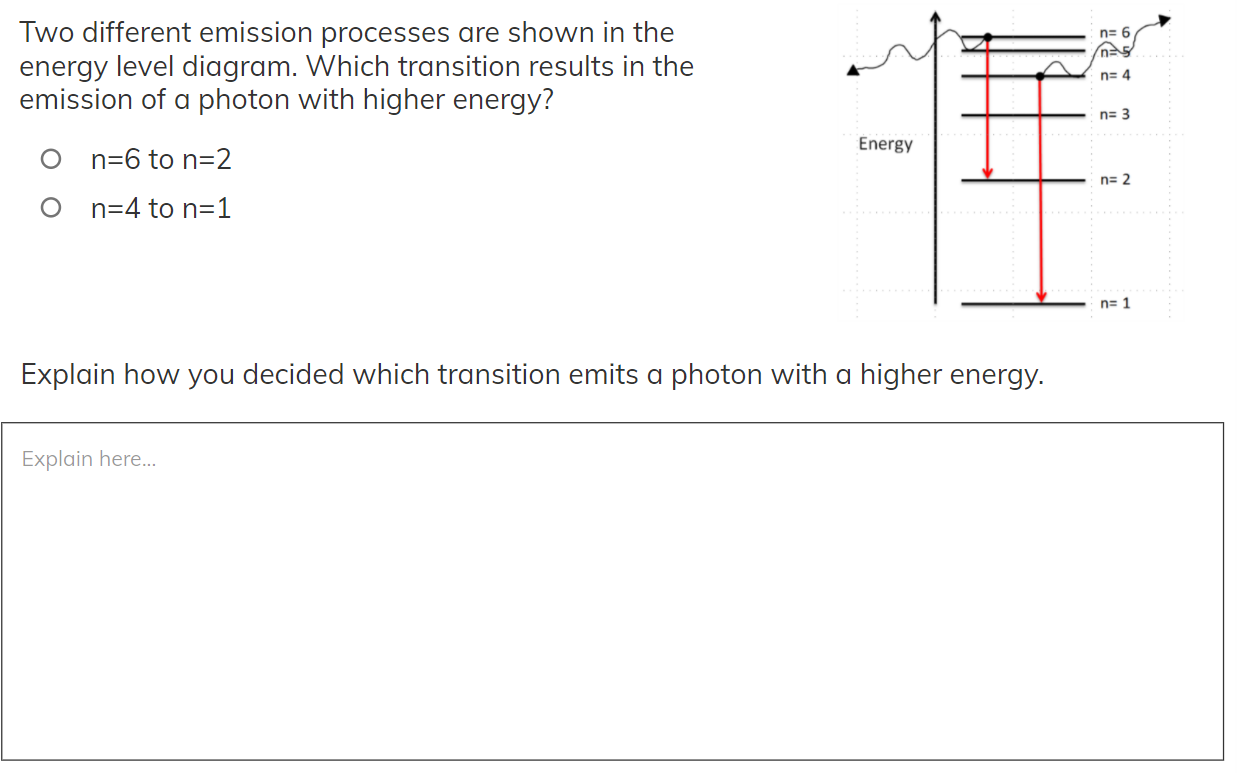
n= 6 Two different emission processes are shown in the energy level diagram. Which transition results in the emission of a photon with higher energy? n=4 n= 3 Energy on=6 to n=2 n= 2 on=4 to n=1 n=1 Explain how you decided which transition emits a photon with a higher energy. Explain here...
没有找到相关结果