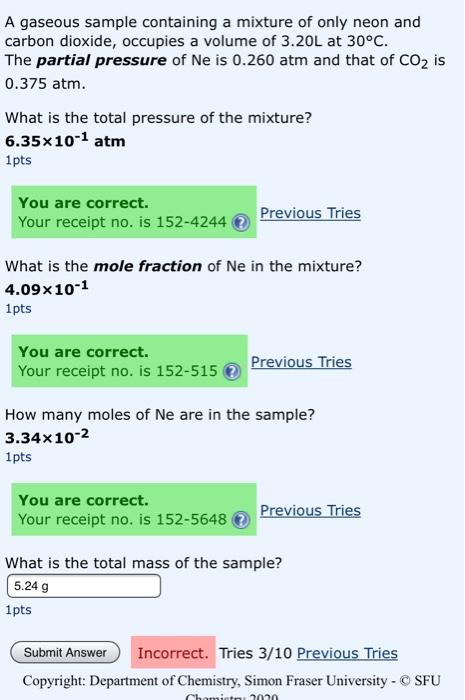
A gaseous sample containing a mixture of only neon and carbon dioxide, occupies a volume of 3.20L at 30°C. The partial pressure of Ne is 0.260 atm and that of CO2 is 0.375 atm. What is the total pressure of the mixture? 6.35x10-1 atm 1pts You are correct. Your receipt no. is 152-4244 Previous Tries What is the mole fraction of Ne in the mixture? 4.09x10-1 1pts You are correct. Your receipt no. is 152-515 Previous Tries How many moles of Ne are in the sample? 3.34x10-2 1pts You are correct. Your receipt no. is 152-5648 Previous Tries What is the total mass of the sample? 5.24 g 1pts Submit Answer Incorrect. Tries 3/10 Previous Tries Copyright: Department of Chemistry, Simon Fraser University - SFU Chomioto 2010
没有找到相关结果