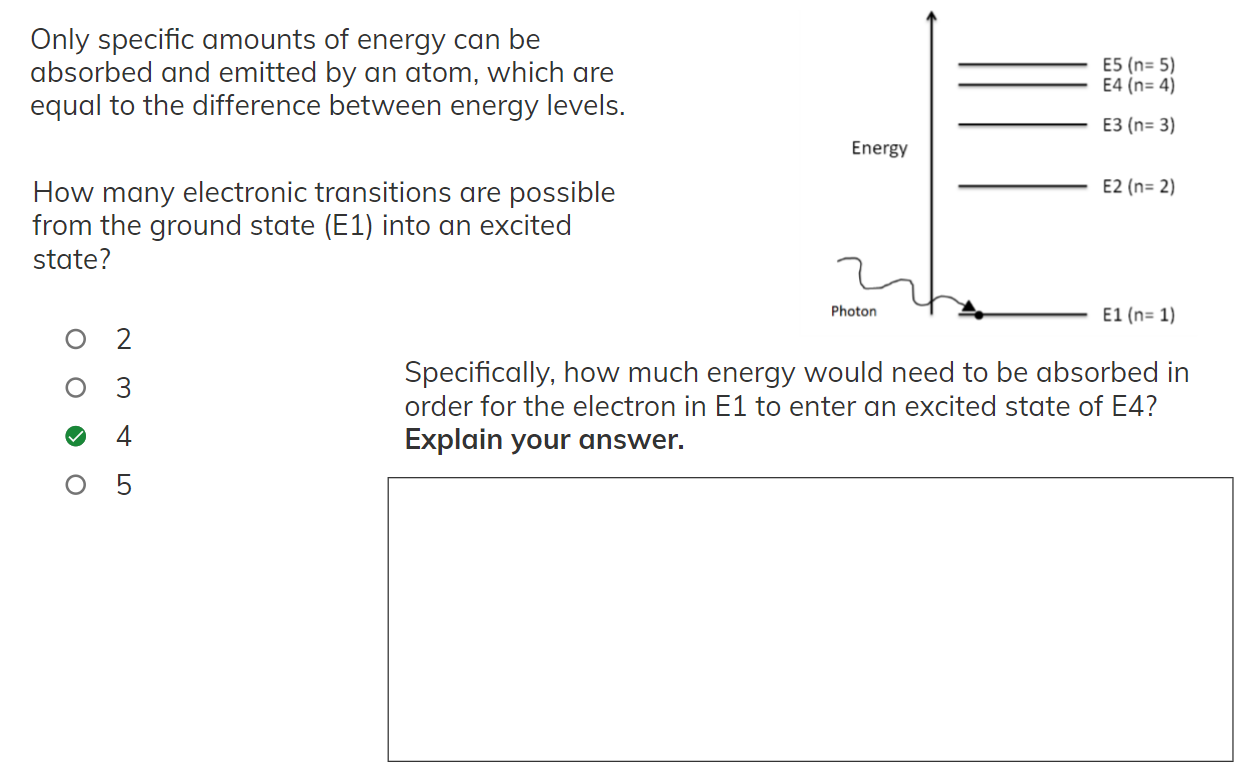
Only specific amounts of energy can be absorbed and emitted by an atom, which are equal to the difference between energy levels. E5 (n=5) E4 (n=4) E3 (n=3) Energy E2 (n=2) How many electronic transitions are possible from the ground state (E1) into an excited state? Photon E1 (n=1) O 2. O 3 Specifically, how much energy would need to be absorbed in order for the electron in E1 to enter an excited state of E4? Explain your answer. 4 O 5
没有找到相关结果