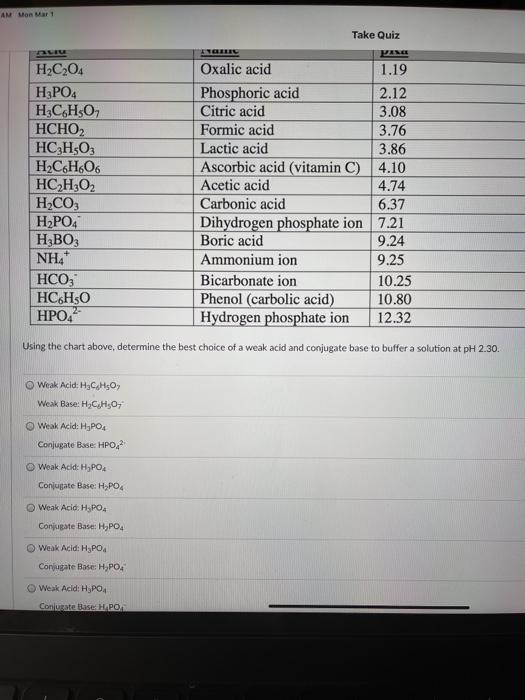
AM Mon Mar 1 Take Quiz H2C204 Oxalic acid 1.19 H3PO4 Phosphoric acid 2.12 H3C6H30 Citric acid 3.08 НСНО, Formic acid 3.76 HC3H503 Lactic acid 3.86 H2C6H606 Ascorbic acid (vitamin C) 4.10 HC2H:02 Acetic acid 4.74 H2CO3 Carbonic acid 6.37 H2PO4 Dihydrogen phosphate ion 7.21 HBO3 Boric acid 9.24 NH4 Ammonium ion 9.25 HCO, Bicarbonate ion 10.25 HC6H50 Phenol (carbolic acid) 10.80 HPO4 Hydrogen phosphate ion 12.32 Using the chart above, determine the best choice of a weak acid and conjugate base to buffer a solution at pH 2.30. Weak Acid: H CHO Weak Base: HC H30 Weak Acid: H,PO4 Conjugate Base: HPO 2 Weak Acid: HjPO4 Conjugate Base: H2PO4 Weak Acid. H.PO Conjugate Base: HPO4 Weak Acid: H3PO4 Conjugate Base H,PO4 Weak Acid: H3PO4 Conturate Base H PO
没有找到相关结果