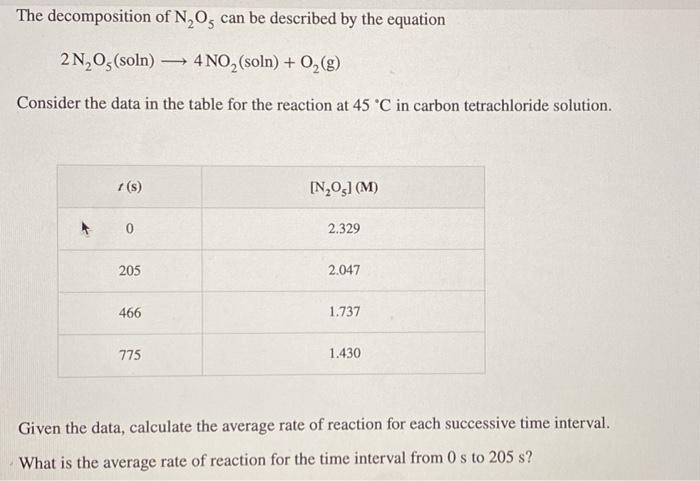
The decomposition of N,Os can be described by the equation 2N,Os(soln) 4 NO, (soln) + 02 (8) Consider the data in the table for the reaction at 45 °C in carbon tetrachloride solution. 1(s) [N,O3] (M) 0 2.329 205 2.047 466 1.737 775 1.430 Given the data, calculate the average rate of reaction for each successive time interval. What is the average rate of reaction for the time interval from Os to 205 s?
What is the average rate of reaction for the time interval from Os to 205 s? average rate of reaction: M/S What is the average rate of reaction for the time interval from 205 s to 466 s? average rate of reaction: M/S What is the average rate of reaction for the time interval from 466 s to 775 s? M/s average rate of reaction:
没有找到相关结果