

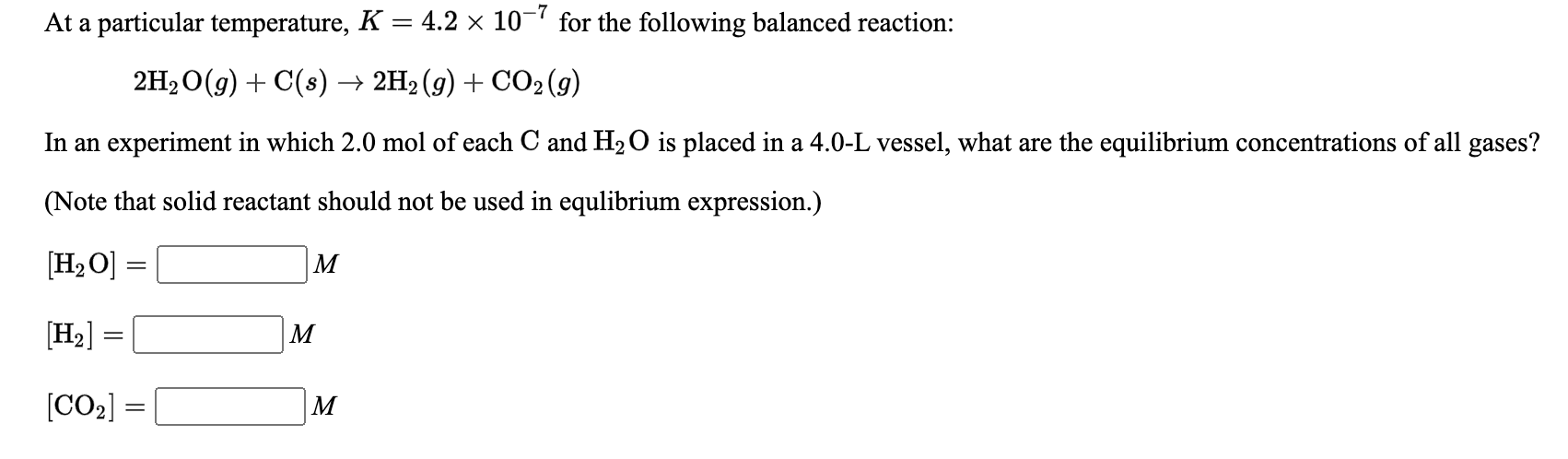
At a particular temperature, K = 2.0 x 10-6 for the following balanced reaction: 2Cl2(g) + O2(g) → 2C12O(g) In an experiment in which 2.5 mol of Cl2 and 2.0 mol of O2 are mixed in a 2.0-L vessel, what are the equilibrium concentrations of all gases? [Cl2] = M [O2] = M (C12O) = M At a particular temperature, K = 6.5 x 10-6 for the following balanced reaction: 2PC13 (g) + O2(g) → 2PC12O(g) In an experiment in which 1.0 mol of PC13 and 1.5 mol of O2 are mixed in a 2.5-L vessel, what are the equilibrium concentrations of all gases? [PC13] = | М [ (O2) = M [PCI:O) = M At a particular temperature, K = 4.2 x 10-7 for the following balanced reaction: 2H2O(g) + C(s) + 2H2(g) + CO2(g) In an experiment in which 2.0 mol of each C and H2O is placed in a 4.0-L vessel, what are the equilibrium concentrations of all gases? (Note that solid reactant should not be used in equlibrium expression.) [H2O] = = M [H2] = M [CO2] ) = M
没有找到相关结果