please answer all the questions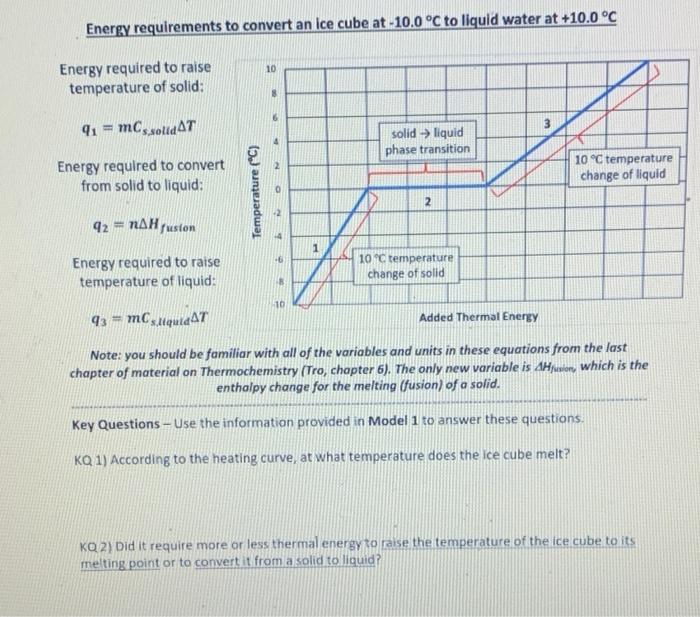
Energy requirements to convert an ice cube at -10.0°C to liquid water at +10.0°C 10 Energy required to raise temperature of solid: 91 = ms solid AT solid -> liquid phase transition Energy required to convert from solid to liquid: 10 °C temperature change of liquid 2 0 Temperature (°C) 2 92 - NAHjuston Energy required to raise temperature of liquid: 10°C temperature change of solid 10 43 - MCsique AT Added Thermal Energy Note: you should be familiar with all of the variables and units in these equations from the last chapter of material on Thermochemistry (Tro, chapter 6). The only new variable is dion, which is the enthalpy change for the melting (fusion) of a solid. Key Questions - Use the information provided in Model 1 to answer these questions KQ 1) According to the heating curve, at what temperature does the ice cube melt? KQ 2) Did It require more or less thermal energy to raise the temperature of the ice cube to its melting point or to convert it from a solid to liquid?
KQ 3) Write the equation used to determine the amount of thermal energy (a) required to raise the temperature of a solid and define all of the variables (including their units) in the equation. 2 KQ 4) Write the equation used to determine the amount of thermal energy (a) required to convert from a solid to a liquid and define all of the variables (including their units) in the equation KQ 5) How is the equation for raising the temperature of a liquid different from that for raising the temperature of a solid?
Exercises - These questions will help you develop your understanding of the relationships presented in Model 1. For each Exercise, report numerical answers with the correct number of significant digits. EX 1) Determine the amount of thermal energy (a) required to raise the temperature of 10.0 g solid water from -10.0°C to its melting point. Cs solid 2.09 / gn EX 2) Determine the amount of thermal energy (a) required to convert 10.0 g solid water to liquid water at its melting point. AHjusion = 6.02 mol EX 3) Determine the amount of thermal energy (9) required to raise the temperature of 10.08 liquid water from its melting point to +10.0°C. Celiquia = 4.18 EX 4) Determine the total amount of thermal energy required to raise 10.0 g of ice at 10.0°C to liquid water at +10.0 C.
没有找到相关结果