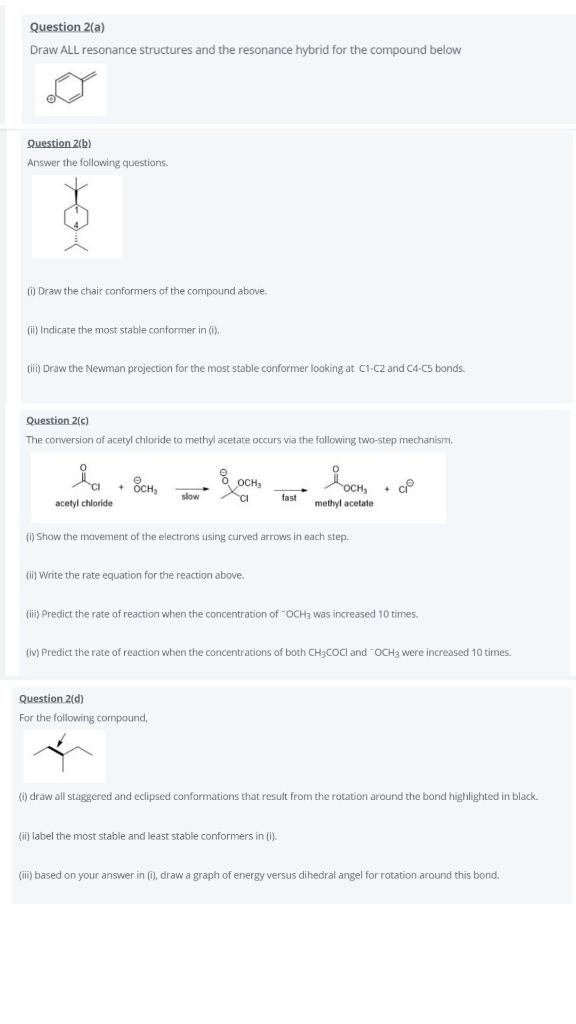
Question 2(a) Draw ALL resonance structures and the resonance hybrid for the compound below Question 2(b) Answer the following questions. (1) Draw the chair conformers of the compound above Indicate the most stable conformer in). clin) Draw the Newman projection for the most stable conformer looking at C1-C2 and C4-c5 bonds. Question 2(0) The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism. id • Всн, OCH, ch + slow -- fast осн, methyl acetate acetyl chloride (1) Show the movement of the electrons using curved arrows in each step (ii) Write the rate equation for the reaction above. (ii) Predict the rate of reaction when the concentration of OCHa was increased 10 times. (lv) Predict the rate of reaction when the concentrations of both CH3COCl and OCH3 were increased 10 times. Question 2d) For the following compound, (1) draw all staggered and eclipsed conformations that result from the rotation around the bond highlighted in black {i) label the most stable and least stable conformers in () (ii) based on your answer in 0, draw a graph of energy versus dihedral angel for rotation around
没有找到相关结果