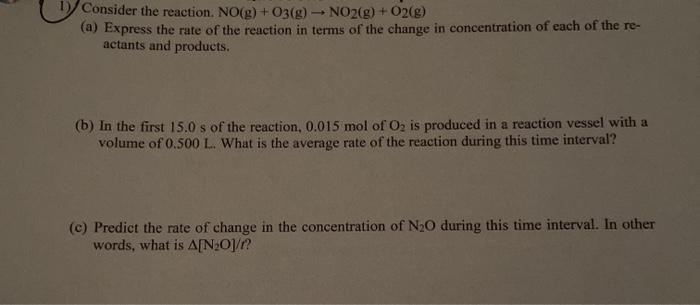
Consider the reaction. NO(g) + O2(g) - NO2(g) + O2(g) (a) Express the rate of the reaction in terms of the change in concentration of each of the re- actants and products. (b) In the first 15.0 s of the reaction, 0.015 mol of O2 is produced in a reaction vessel with a volume of 0.500 L. What is the average rate of the reaction during this time interval? (©) Predict the rate of change in the concentration of N20 during this time interval. In other words, what is A[NO]/?
没有找到相关结果