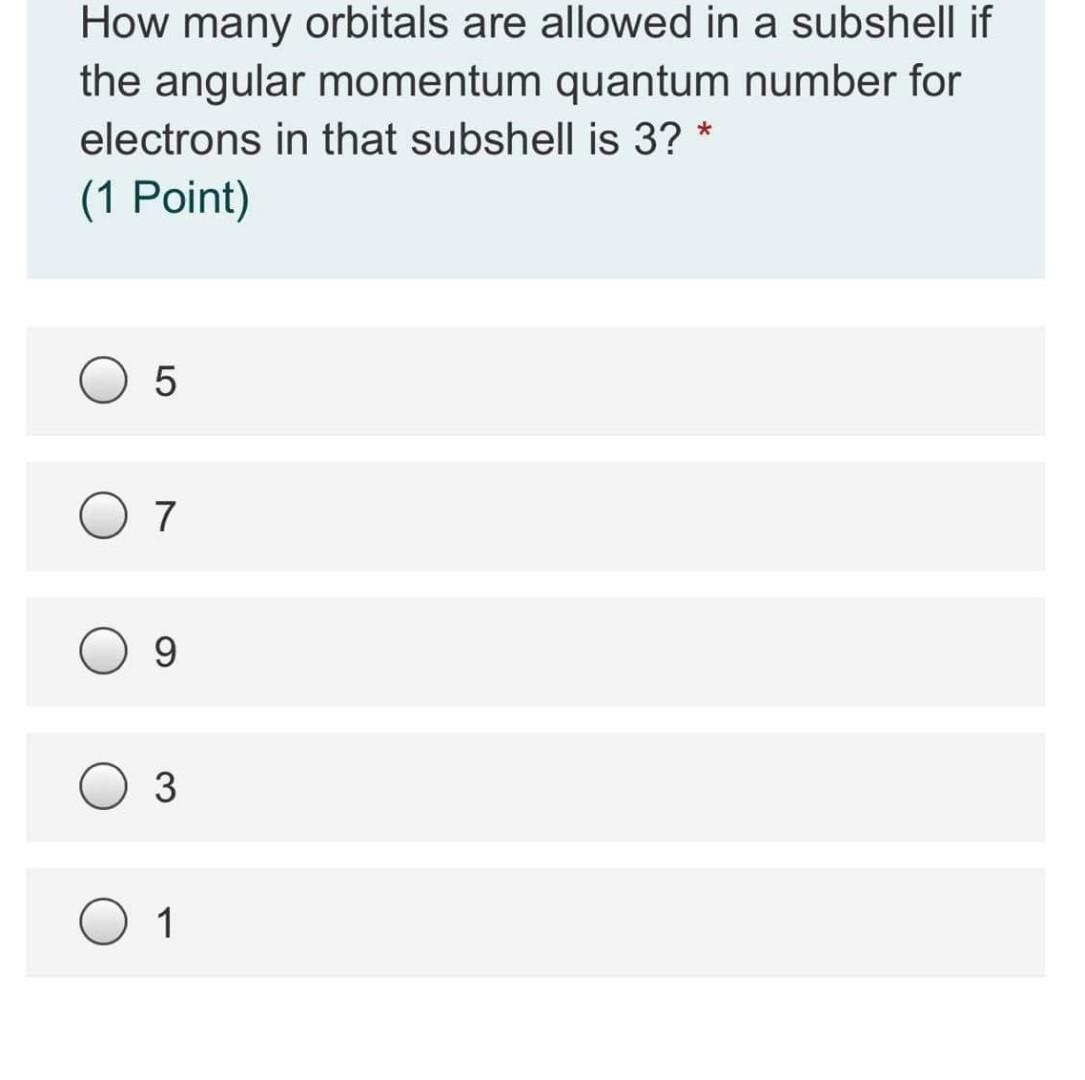
How many orbitals are allowed in a subshell if the angular momentum quantum number for electrons in that subshell is 3? * (1 Point) 5 7 9 3 1 The electron configuration of a ground-state vanadium atom (V) is (1 Point) 1 18 1 2 He 4.0026 1.008 2 13 15 16 17 9 18.998 17 CI 35.45 35 3 4 5 8 9 32.06 26 21 Sc 44.956 47.867 4 Li Be 6.94 90122 11 12 Na Mg 22.990 24.305 19 20 K Ca 39 098 40.078 37 38 Rb Sr 85.46887.62 55 56 Cs Ba 132.91 13733 87 88 Fr Ra (223) (226) 50 942 6 7 24 25 Mn 51.946 34918 42 43 Mo Tc 95.95 (98) 74 75 W Re 183.86 186.21 106 107 Sg Bh (271) (270) 55.845 44 Ru 10107 10 Ne 20.180 18 Ar 39.948 36 Kr 83.798 54 Xe 131.29 86 Rn 6 B c 10.81 12011 13 14 AI Si 26.982 28.085 31 32 Ga Ge 69.723 72,630 49 50 In Sn 11482 118.71 81 TI Pb 204.38 2072 113 114 Nh FI (286) (289) 27 Co 58.933 45 Rh 102.91 77 Ir 192.22 109 Mt (276) Nb 92.906 73 7 8 N O 14.007 15.999 IS 16 P S 30.974 33 As Se 74.922 78.97 51 52 Sb Te 121.76127.60 83 84 ві Po 208.98 HIS 116 Mc Lv (289) (293) 10 11 12 28 29 30 Ni Cu Zn 58.693 63 546 65.38 46 47 48 Pd Ag Cd 106 42 10787 112.41 78 79 80 Pt Au Hg 195 OR 196 97 200 59 110 111 112 Ds Rg Cn (281) (280) (285) 39 40 Zr 88.90691.224 57-71 72 HE 178.39 89.103 104 # Rr (265) 79.904 53 1 126.90 85 At (210) 117 Ts (294) Os 190.23 (209) (222) 180.95 105 Db (268) 108 Hs (277) 118 Og (294) • Lanthanide series 59 60 Pr Nd 140.91 144.24 61 Pm 63 Sm Eu Gd 150.36 151.96 15725 65 Tb 158.93 66 Dy 162 50 Ho 164.93 68 Er 167.26 69 Tm 168.93 70 Yb 173.05 Lu 174.97 57 58 Ce 13891140.12 89 90 Ac Th 77 # Actinide series 91 Pa 92 U 93 Np . Pu 7441 95 Am 96 Cm 1 ВК 98 cr 16 Es 100 Fm 101 Md 102 No 103 Lr O [Ar]4524d3. [Ar]452 4p3. [Ar]4s23d3. O [Ar]3d.
没有找到相关结果