please help with these 2 questions asap!
For the second question, the protocol for the experiment isbelow the data. These are all the information I am given.
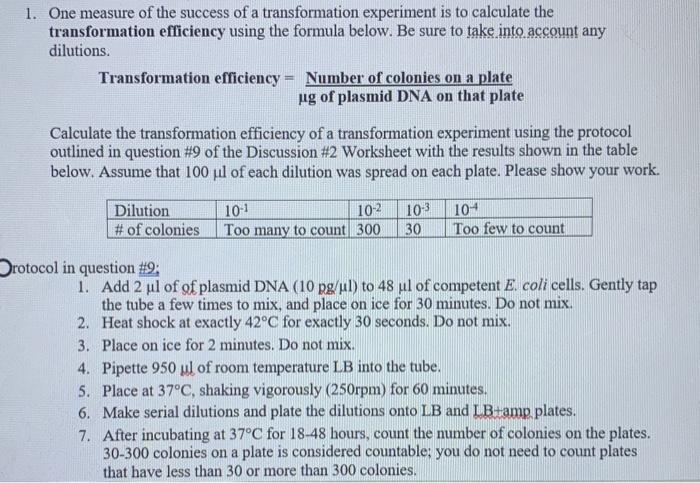
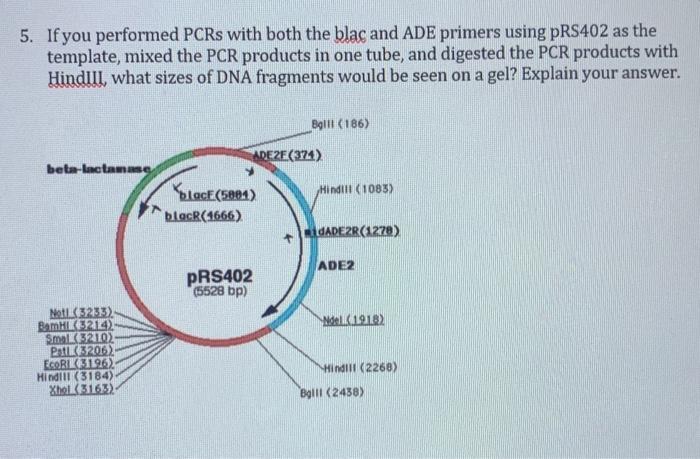
5. If you performed PCRs with both the blac and ADE primers using pR$402 as the template, mixed the PCR products in one tube, and digested the PCR products with Hindill, what sizes of DNA fragments would be seen on a gel? Explain your answer. Boll (186) ARE ZE(374) beta-lactang Place(5891) Hindilt (1083) blacR(1666) REGADE2R(1278) ADE2 PRS402 (5528 bp) Nel 1918) Noti( 32313) BamH214) Small(3210) Pati 32062 EcoRIL 31962 Hindill (3184) Xhel (3163) Hindill (2268) Boll (2438) 1. One measure of the success of a transformation experiment is to calculate the transformation efficiency using the formula below. Be sure to take into account any dilutions. Transformation efficiency = Number of colonies on a plate ug of plasmid DNA on that plate Calculate the transformation efficiency of a transformation experiment using the protocol outlined in question #9 of the Discussion #2 Worksheet with the results shown in the table below. Assume that 100 uil of each dilution was spread on each plate. Please show your work. Dilution # of colonies 10-1 10-2 Too many to count 300 10-3 30 10+ Too few to count Protocol in question #9: 1. Add 2 ul of of plasmid DNA (10 pg/ul) to 48 ul of competent E. coli cells. Gently tap the tube a few times to mix, and place on ice for 30 minutes. Do not mix. 2. Heat shock at exactly 42°C for exactly 30 seconds. Do not mix. 3. Place on ice for 2 minutes. Do not mix. 4. Pipette 950 ul of room temperature LB into the tube. 5. Place at 37°C, shaking vigorously (250rpm) for 60 minutes. 6. Make serial dilutions and plate the dilutions onto LB and I B+amp plates. 7. After incubating at 37°C for 18-48 hours, count the number of colonies on the plates. 30-300 colonies on a plate is considered countable: you do not need to count plates that have less than 30 or more than 300 colonies.
没有找到相关结果