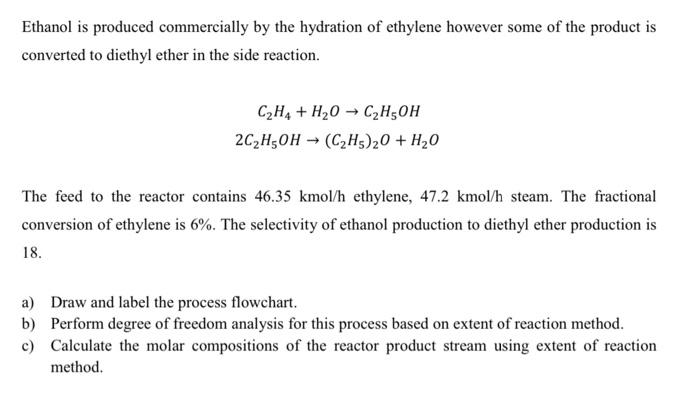
Ethanol is produced commercially by the hydration of ethylene however some of the product is converted to diethyl ether in the side reaction. C2H4 + H2O → C2H50H 2C2H5OH → (C2H5)20 + H2O The feed to the reactor contains 46.35 kmol/h ethylene, 47.2 kmol/h steam. The fractional conversion of ethylene is 6%. The selectivity of ethanol production to diethyl ether production is 18. a) Draw and label the process flowchart. b) Perform degree of freedom analysis for this process based on extent of reaction method. c) Calculate the molar compositions of the reactor product stream using extent of reaction method.
没有找到相关结果