PLEASE HELP all i will give u like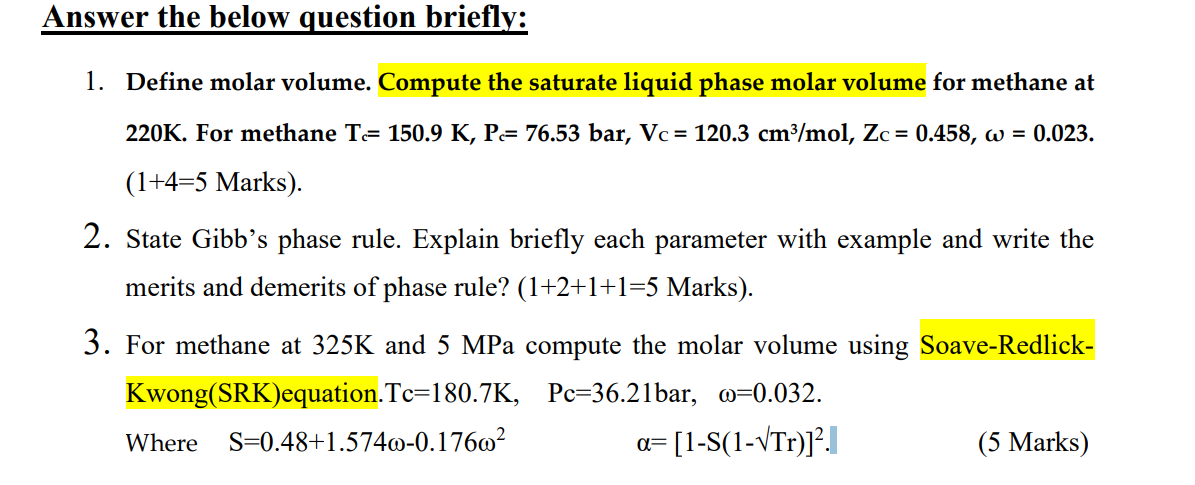
Answer the below question briefly: 1. Define molar volume. Compute the saturate liquid phase molar volume for methane at 220K. For methane T= 150.9 K, P= 76.53 bar, Vc = 120.3 cm3/mol, Zc = 0.458, w = 0.023. (1+4=5 Marks). 2. State Gibb's phase rule. Explain briefly each parameter with example and write the merits and demerits of phase rule? (1+2+1+1=5 Marks). 3. For methane at 325K and 5 MPa compute the molar volume using Soave-Redlick- Kwong(SRK)equation.Tc=180.7K, Pc=36.21bar, m=0.032. Where S=0.48+1.5740-0.17602 a= [1-S(1-VTr)]2.1 (5 Marks)
没有找到相关结果