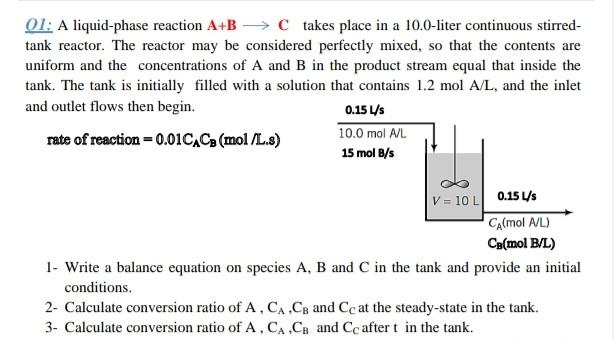
01: A liquid-phase reaction A+B → takes place in a 10.0-liter continuous stirred- tank reactor. The reactor may be considered perfectly mixed, so that the contents are uniform and the concentrations of A and B in the product stream equal that inside the tank. The tank is initially filled with a solution that contains 1.2 mol A/L, and the inlet and outlet flows then begin. 0.15 4S rate of reaction = 0.01C.Co (mol/L.8) 10.0 mol A/L 15 mol B/s V = 10L) 0.15 45 CA(mol A/L) Ca(mol B/L) 1- Write a balance equation on species A, B and C in the tank and provide an initial conditions. 2- Calculate conversion ratio of A, CA.CB and Cc at the steady-state in the tank. 3- Calculate conversion ratio of A.CA.Cg and Cc aftert in the tank.
没有找到相关结果