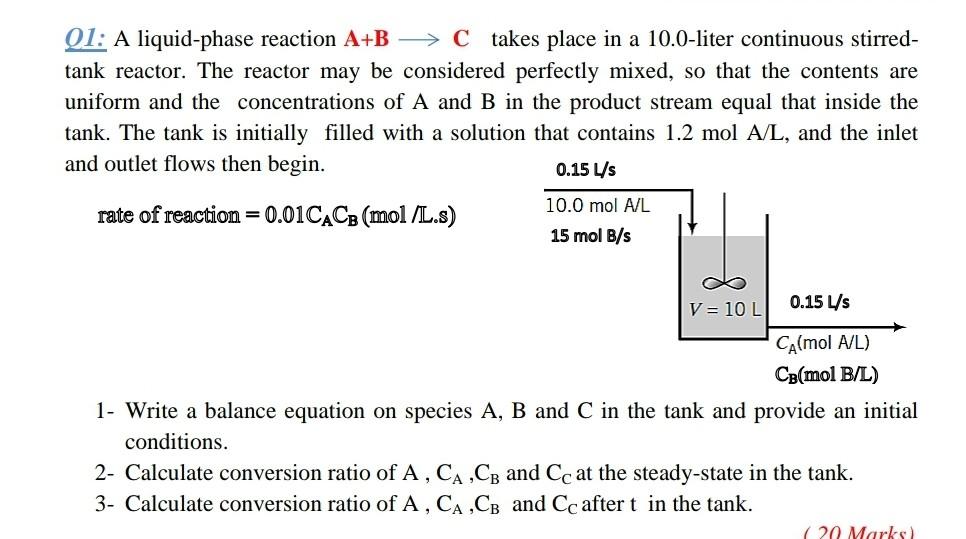
Q1: A liquid-phase reaction A+B → C takes place in a 10.0-liter continuous stirred- tank reactor. The reactor may be considered perfectly mixed, so that the contents are uniform and the concentrations of A and B in the product stream equal that inside the tank. The tank is initially filled with a solution that contains 1.2 mol A/L, and the inlet and outlet flows then begin. 0.15 Us rate of reaction=0.01CACB (mol /L.s) 10.0 mol A/L 15 mol B/s V = 10L 0.15 4s CA(mol A/L) CB(mol B/L) 1- Write a balance equation on species A, B and C in the tank and provide an initial conditions. 2- Calculate conversion ratio of A , CA,CB and Cc at the steady-state in the tank. 3- Calculate conversion ratio of A, CA CB and Cc after t in the tank. 1 20 Marks)
没有找到相关结果