 I am looking for help on partB of this question!
I am looking for help on partB of this question!
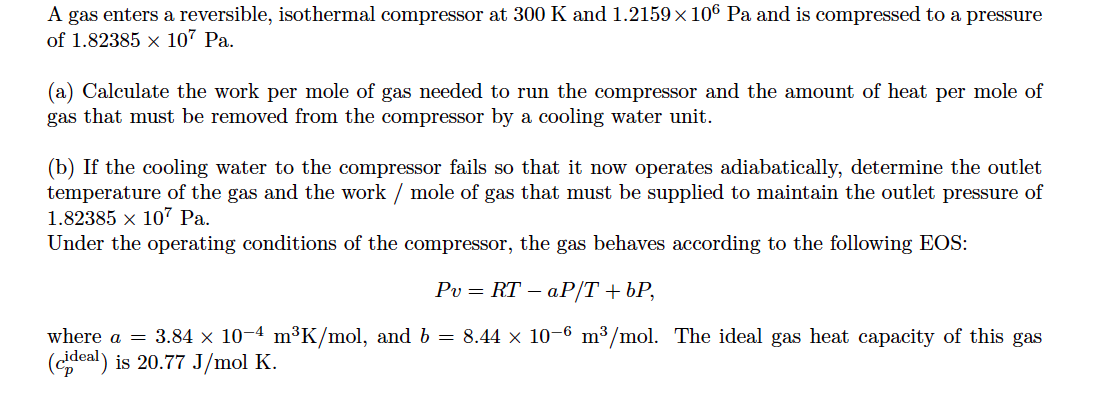
A gas enters a reversible, isothermal compressor at 300 K and 1.2159 x 106 Pa and is compressed to a pressure of 1.82385 x 107 Pa. (a) Calculate the work per mole of gas needed to run the compressor and the amount of heat per mole of gas that must be removed from the compressor by a cooling water unit. (b) If the cooling water to the compressor fails so that it now operates adiabatically, determine the outlet temperature of the gas and the work / mole of gas that must be supplied to maintain the outlet pressure of 1.82385 x 107 Pa. Under the operating conditions of the compressor, the gas behaves according to the following EOS: Pu= RT – aP/T +bP, where a = 3.84 x 10-4 mºK/mol, and b = 8.44 x 10-6 m3/mol. The ideal gas heat capacity of this gas (cideal) is 20.77 J/mol K.
没有找到相关结果