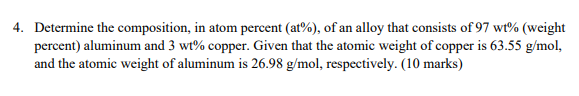
4. Determine the composition, in atom percent (at%), of an alloy that consists of 97 wt% (weight percent) aluminum and 3 wt% copper. Given that the atomic weight of copper is 63.55 g/mol, and the atomic weight of aluminum is 26.98 g/mol, respectively. (10 marks)
没有找到相关结果