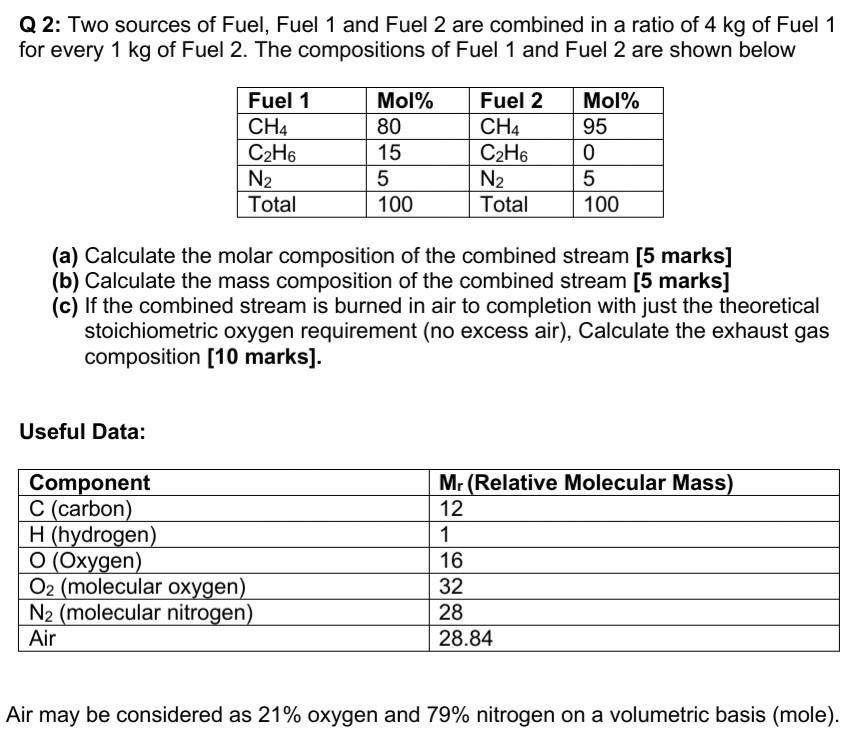
Q2: Two sources of Fuel, Fuel 1 and Fuel 2 are combined in a ratio of 4 kg of Fuel 1 for every 1 kg of Fuel 2. The compositions of Fuel 1 and Fuel 2 are shown below Fuel 1 CH4 C2H6 N2 Total Mol% 80 15 5 100 Fuel 2 CH4 C2H6 N2 Total Mol% 95 0 5 100 (a) Calculate the molar composition of the combined stream [5 marks] (b) Calculate the mass composition of the combined stream [5 marks] (c) If the combined stream is burned in air to completion with just the theoretical stoichiometric oxygen requirement (no excess air), Calculate the exhaust gas composition [10 marks). Useful Data: Component C (carbon) H (hydrogen) O (Oxygen) O2 (molecular oxygen) N2 (molecular nitrogen). Air Mr (Relative Molecular Mass) 12 1 16 32 28 28.84 Air may be considered as 21% oxygen and 79% nitrogen on a volumetric basis (mole).
没有找到相关结果