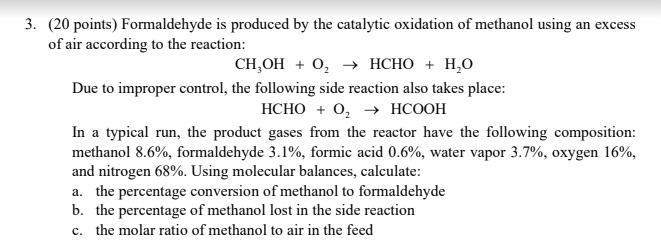
3. (20 points) Formaldehyde is produced by the catalytic oxidation of methanol using an excess of air according to the reaction: CH,OH + 0 + HCHO + H2O Due to improper control, the following side reaction also takes place: HCHO + O → HCOOH In a typical run, the product gases from the reactor have the following composition: methanol 8.6%, formaldehyde 3.1%, formic acid 0.6%, water vapor 3.7%, oxygen 16%, and nitrogen 68%. Using molecular balances, calculate: a. the percentage conversion of methanol to formaldehyde b. the percentage of methanol lost in the side reaction c. the molar ratio of methanol to air in the feed
没有找到相关结果