
Why is 0.1 used in the underlined part of the answer? Shouldn't0.2 be used instead? Please explain
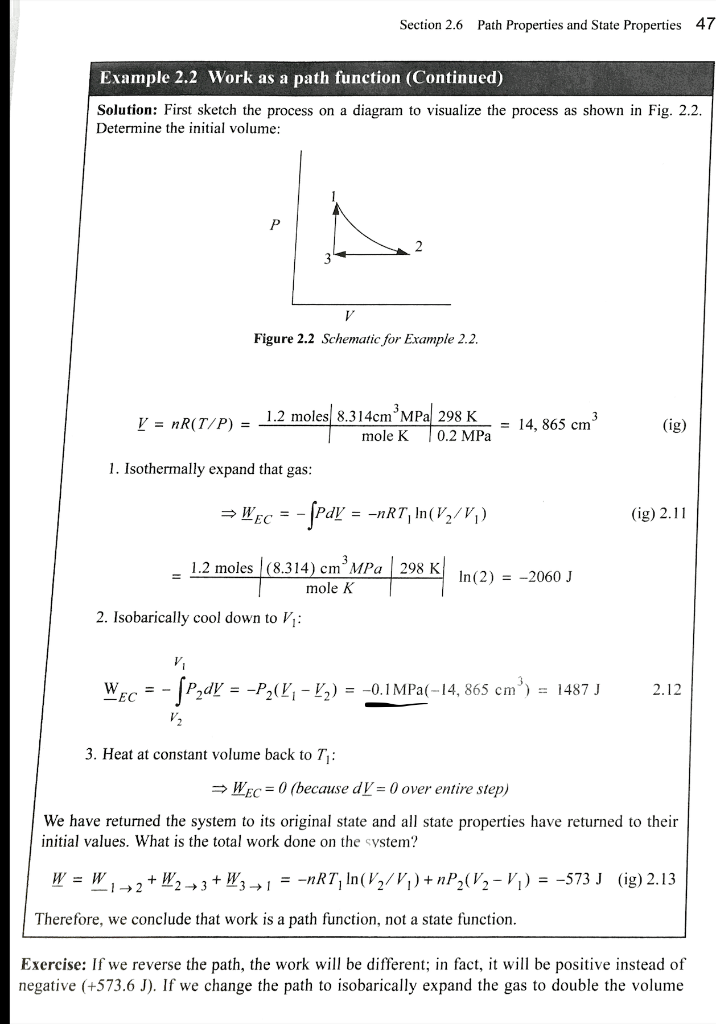
Example 2.2 Work as a path function Consider 1.2 moles of an ideal gas in a piston at 298 K and 0.2 MPa and at volume 1. The gas is expanded isothermally to twice its original volume, then cooled isobarically to Kl. It is then heated at constant volume back to T. Demonstrate that the net work is non-zero, and that the work depends on the path. Section 2.6 Path Properties and State Properties 47 Example 2.2 Work as a path function (Continued) Solution: First sketch the process on a diagram to visualize the process as shown in Fig. 2.2. Determine the initial volume: P V Figure 2.2 Schematic for Example 2.2. V = nR(T/P) = 1.2 moles 8.314cm MPa 298 K mole K 0.2 MPa = 14, 865 cm (ig) 1. Isothermally expand that gas: = Wec = - ſpay = =nRT, In(V2/V;) (ig) 2.11 1.2 moles (8.314) cm MPa 298 K mole K In(2) = -2060 J 2. Isobarically cool down to V: Wec = -5P2dy = -P2(4, - ) = -0.1MPa(-14, 865 cm") = 1487 J jener 2.12 3. Heat at constant volume back to T1 : Wec = 0 (because dV = 0 over entire step) We have returned the system to its original state and all state properties have returned to their initial values. What is the total work done on the system? W = W, 12 + W2 +3 + W3+1 = -nRT, In(V2/V1) +nP (V2-1) = -573 J (g) 2.13 Therefore, we conclude that work is a path function, not a state function. Exercise: If we reverse the path, the work will be different; in fact, it will be positive instead of negative (+573.6 J). If we change the path to isobarically expand the gas to double the volume
没有找到相关结果