PLEASE SOLVE BOTH A & B
SUBJECT : CHEMICAL REACTION ENGINEERING
PLEASE SHOW THE FULL METHOD WITH EXPLANATION
THANKYOU
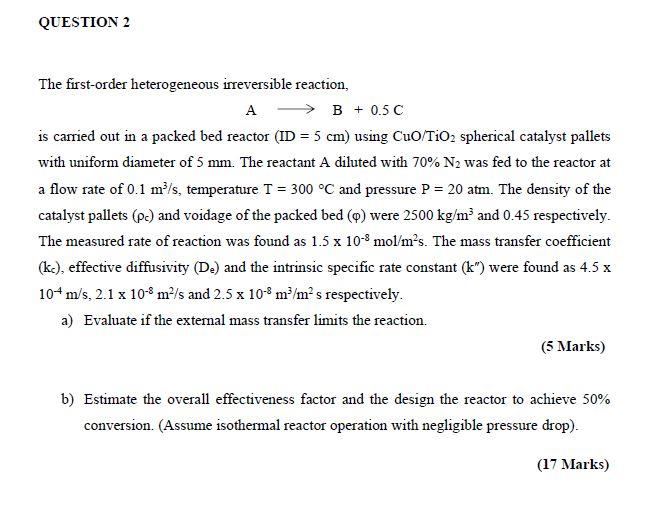
QUESTION 2 The first-order heterogeneous irreversible reaction, A - B +0.5C is carried out in a packed bed reactor (ID = 5 cm) using CuO/TiO2 spherical catalyst pallets with uniform diameter of 5 mm. The reactant A diluted with 70% N2 was fed to the reactor at a flow rate of 0.1 m/s, temperature T = 300 °C and pressure P = 20 atm. The density of the catalyst pallets (Pc) and voidage of the packed bed (m) were 2500 kg/m and 0.45 respectively. The measured rate of reaction was found as 1.5 x 10-8 mol/mºs. The mass transfer coefficient (ke), effective diffusivity (D.) and the intrinsic specific rate constant (k”) were found as 4.5 x 104m/s, 2.1 x 10-8 m²/s and 2.5 x 10-8 m/mºs respectively. a) Evaluate if the external mass transfer limits the reaction. (5 Marks) b) Estimate the overall effectiveness factor and the design the reactor to achieve 50% conversion. (Assume isothermal reactor operation with negligible pressure drop). (17 Marks)
没有找到相关结果