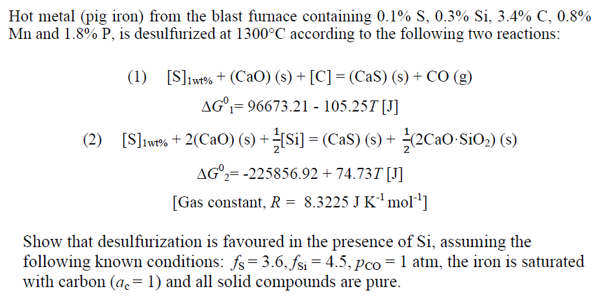
Hot metal (pig iron) from the blast furnace containing 0.1% S, 0.3% Si, 3.4% C, 0.8% Mn and 1.80% P, is desulfurized at 1300°C according to the following two reactions: (1) [S] wt% + (CaO) (s) +[C]=(CaS) (s)+ CO (g) AG°= 96673.21 - 105.25T [J] (2) [SJws + 2(CaO) (s) + (si]=(CaS) (s) + 2Cao SiO2) (s) AG°2=-225856.92 + 74.737 [J] [Gas constant, R = 8.3225 J K 'mol'] Show that desulfurization is favoured in the presence of Si, assuming the following known conditions: fs = 3.6, fsi = 4.5, pco = 1 atm, the iron is saturated with carbon (ac = 1) and all solid compounds are pure.
没有找到相关结果